From 01.01.2022: DEMIS interface will be mandatory
From 01.01.2022, laboratories are obliged to report all notifiable infections to health authorities and RKI via their DEMIS interface. This should relieve laboratories and health authorities. If you do not yet have a DEMIS interface in your LIS, we will be happy to inform you at vertrieb@medat.de.
Since June 2020, laboratories have been able to electronically report pathogen detection of SARS-CoV-2 to the responsible health authorities. Thanks to the electronic notification, the information on SARS-CoV-2 infections is available more quickly and more fully to the health department, and infection protection measures can be initiated promptly to prevent further infections.
The data can be sent from your laboratory information system to the DEMIS adapter. This translates the content into the HL / -FHIR format used by DEMIS. The reports are then validated in the central system and sent to the responsible health department in encrypted form.
According to the RKI, the aim of the DEMIS project is to further develop and improve the existing reporting system for infectious diseases in accordance with the Infection Protection Act (IfSG).
Archiving and exchange interface (AWS)
An archiving and exchange interface (AWS) of the practice management systems (PSV) has been mandatory since the beginning of June 2021. The KBV has defined an interface for archiving and changing the practice management system and published it on June 6, 2019 in the vesta interoperability directory.
Medat has implemented the required AWS in all systems, the correct implementation has been confirmed by the KBV. The interface is based on the international, cross-sector and open HL7 FHIR standard.
This interface makes it easier for practices to change the practice administration system. In addition, practices can archive patient data independently of their practice management system.
Medat at trade fairs
The 20th annual conference of the Association of Accredited Laboratories starts this week(AAL). On 24th and 25th of September 2021 the following main topics are dealt with in Augsburg:
- IVDR: Effects on “lab developed tests” and lessons from the corona pandemic
- Digital applications in the laboratory between KRITIS, GDPR and IVDR
- Genetic Diagnostics Act Validation of Human Genetic Methods “
- “Innovations DIN 15189, RiliBÄK, DAkkS”
There is currently still free capacity to participate. Registration is possible at https://www.aal-tagung.de/anmeldung. You can find us at booth no.14.
In addition, Medat is taking part in this year’s Euromedlab. Organized by the German Society for Clinical Chemistry and Laboratory Medicine (DGKL), the congress will take place from November 28th to December 2nd in Munich. The Euromedlab conference offers Medat the opportunity to exchange ideas with international speakers about the latest developments in laboratory medicine.
The corona pandemic has had a profound impact on our social life and our health system since the beginning of 2020. This also showed us how important the work is in our field of laboratory diagnostics and software. For this reason, we are also taking part this year from 02. until 4th of December at the 20th KMIS with the focus on “Diagnostics, management and vaccinations against SARS-CoV-2 infections” in Berlin. In addition to the corona pandemic, other important and overarching topics are also dealt with, such as current guidelines, fungal and difficult-to-treat infections and immunomodulatory therapies.
Successful trade fair during the pandemic – review DMEA 2021
The first virtual DMEA fair took place in June. With a digital trade fair presence including an interactive trade fair tour, Medat answered the trade fair participants’ questions about our laboratory information system. The special topics we offer such as order entry, human genetics and system security (KRITIS) have met with great demand. For everyone who would like to experience the program again: all program items are available on demand for ticket holders until September 12th.
We would like to thank all interested participants and the many interesting conversations!
After a purely digital trade fair, we look forward to being there for you again next year. Because: the date for the next DMEA is already known. The fair will take place in Berlin from April 26th to 28th, 2022.
Medat is also represented at other trade fairs this year:
At the Euromedlab in Munich (November 28th – December 2nd, 2021) and at KMIS in Berlin (December 2nd – 4th, 2021).
You will find more information about the trade fairs on our website soon.
Did you know that …? – Medat on Social Media
Did you know that Medat is represented on the social networks Facebook and Instagram? Here you get the latest news, insights behind the scenes and information about Medat. And Medat is also represented on Google: You can find the latest articles in your Google search for Medat.
The expansion of our website on Facebook, Instagram and Google is part of the digital realignment of Medat, which began with the launch of the new website in May 2020.
Our repositioning will be continued this year with a new image video, several virtual reality tours and next year an in-house Medat academy.
Follow us on Facebook https://www.facebook.com/medatlaborinformationssystem and on Instagram https://www.instagram.com/medatlaborinformationssystem/
Medat takes position
Managing Director Stefan Henkelmann is pleased about the start of the completely new website:
“Both our customers and our employees as well as new customers should have the opportunity on the new homepage to find out more about Medat’s laboratory information system. The various modules such as order entry, blood depot or human genetics as well as the advantages of the LIS are presented.
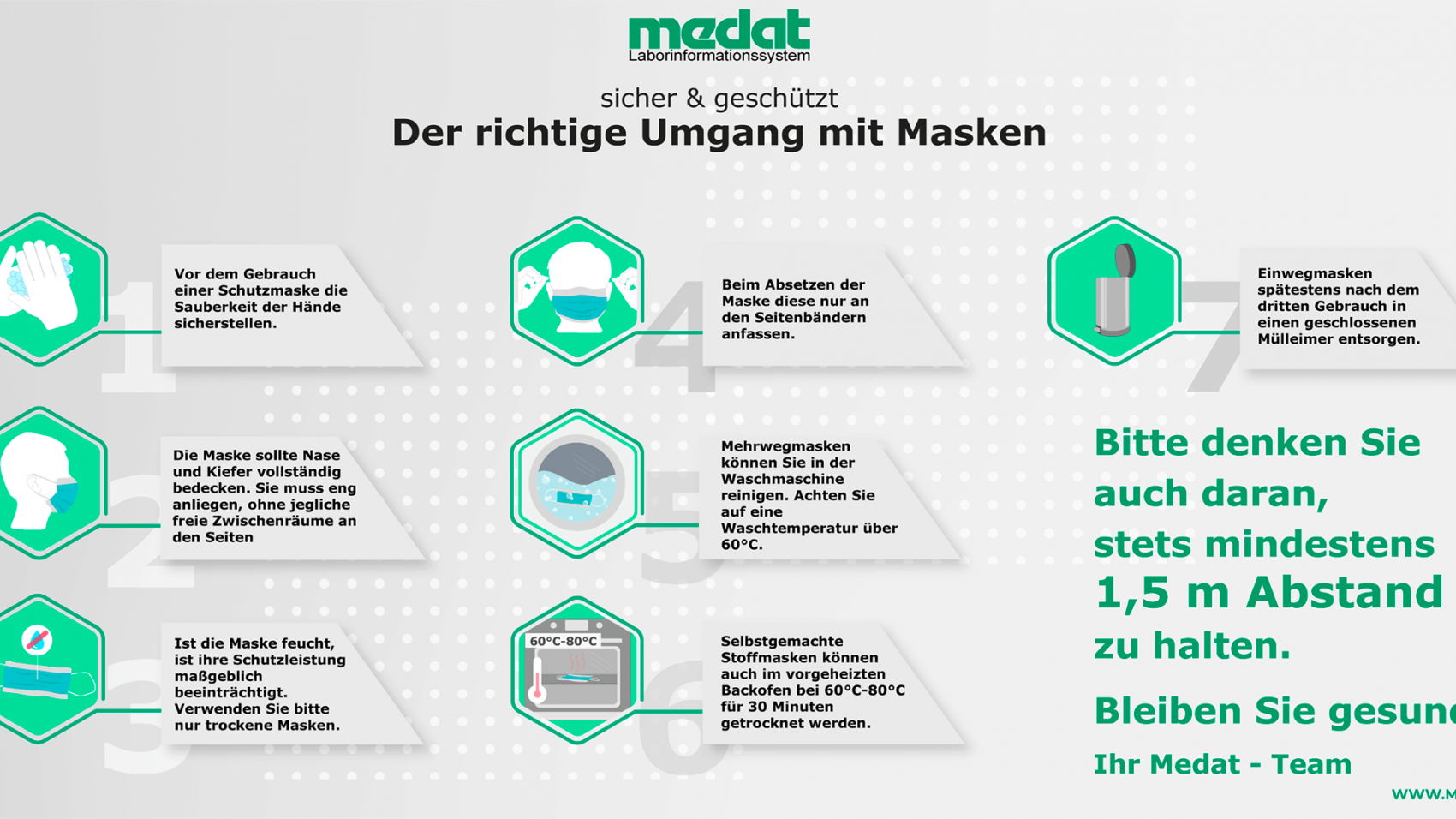
We will publish further news articles on the homepage at regular intervals. One post includes, for example, the correct handling of wearing a face mask.
In addition, there will be a newsletter every three months which will provide information on current topics.
The first newsletter is expected to appear at the end of June 2020.
The new homepage was designed and implemented by the marketing experts at ST Design.
In the near future there will be the Medat Academy. Both Medat employees and external employees will be able to access the online academy.
The start will be the first quarter of 2021.
Managing Director of ST Design and Lecturer for Online Marketing Sascha Tominschek explains the concept behind the new look:
“With the realignment of Medat, the corporate identity has been revised in a conceptually innovative way.
We are pleased to present you the new website as the first unit of this overall concept.
The Medat website sets new standards in the area of design and user guidance. It makes the versatile laboratory information system tangible and transparent.
The profitability of the individual modules is broken down in simple steps for the management level, the laboratory doctors and IT.
Furthermore, we work in close cooperation with the specialists from Medat to ensure that customers can use the software even more easily and intuitively. “
Medat Computer-Systeme GmbH
Medat has been a medium-sized, independent and owner-managed company in the field of laboratory information systems for over 45 years.
Our employees guarantee existing and new customers optimal service and support.
The company takes care of over 100 satisfied customers in Germany and Austria.
With currently approx. 4000 connected measuring cells, including distribution robots, standalone devices and road systems from a program collection of meanwhile more than 150 different device drivers.
The system offers its own order entry with optimal support for preanalytics.
Reports are available in multiple languages.
Our customers include private laboratories, laboratory communities, laboratory specialists, universities and hospitals.
Medat is a member of the German Society for Clinical Chemistry and Laboratory Medicine, as well as the quality ring for medical software, and in the working groups LDT3 and 1LV.
The company is certified according to the quality management standard DIN EN ISO 9001.
Changeover of VAT from 01.07.2020 – Important customer information
We have discussed the subject of “sales tax” internally and can provide you with a solution for private billing 1.7.2020 (ASCII, XML) if required. This consists of an update and an additional table to be maintained by your company, in which the information relevant for versions is stored in five columns (example see below). The VAT must be entered here in the cost unit. We need an own cost unit.
This definition table will also be used for the collective invoice. In all probability, we will be able to provide an update with a solution in mid-July at the earliest. The collective invoice program will use the same table for versioning the VAT rates. Please determine, independently of us, the cost units you are using, which are generated with a VAT rate and are used for VAT-charged invoices (collective, private invoices). If you do not have this yet, please create the cost units with us. There should be an entry in your table for each cost unit (analogous to the last three sample lines).
In the current program versions for 3.7, it will not yet be possible to mix different tax rates (19%, 16%) on one invoice. Therefore, the relevant billing periods should be selected accordingly if this condition occurs. The respective program will check for this condition and should terminate with a message if such a condition occurs.
We would like to draw your attention to the fact that the offsetting of VAT occurs most frequently in the collective invoice. There, however, the date is printed appropriately delimited anyway. Only latecomers in billing (subsequent change from “private” to “collective bill) can cause problems here and force a separate bill after the transition.
This means: You must always enter “07/01/2020” for future collective invoices with the “from date”.
Latecomers from previous quarters must then be billed separately, for example through “01/01/2020 – 06/30/2020”.
Private orders that have to be charged with VAT are usually individual orders that do not result in any excess within an invoice. Private invoices with different tax rates (based on the time of acceptance or import) processed within a billing run (XML private billing: “Ticket”) are no problem and should be appropriately assigned to the versioning.
If the order data of the same private invoice requires different tax rate versions, the program will issue a warning (XML private billing: red cross at the end of the ticket line), name the patient with errors in the error text, exclude them from the current billing, but generally continue the billing.
The patient must then be treated separately over a time division.
Please also clarify with your accounting department what exactly you need for the changeover.
We ask you to carry out the tests of the programs necessary for the update as soon as possible and on your own, as the short deadline requires quick action.
Changes to layouts will probably also be necessary in which the tax rate appears as a text constant in the layout. This applies to all billing variants. These text constants are replaced by the tax rates used in the case.
Our accounting colleagues will be happy to answer any questions you may have.
GOfax.IP – pilot project by Medat and the Gonicus company
In a pilot project by Medat and Gonicus, the GOfax.IP module was successfully implemented and put into routine operation with our joint customer Labor Berlin Charité Vivantes GmbH.
With this implementation it is possible to combine the topics of e-mail and fax with modern IT. Above all, the module offers laboratory operators economical and reliable communication, as well as seamless integration into the laboratory information system through open standards. The technology behind the GOfax.IP module is a greatly reduced FreeSwitch installation for processing the SIP protocol and receiving the IP fax. The processed data is then transmitted to HylaFAX in the appropriate format. This virtualizes classic fax servers and creates scalable HA environments.
Even if fax has apparently lost its importance in the age of digitization, a closer look shows that it is still widely used in everyday business life. Due to the current corona situation, for example, thousands of findings were faxed to the health authorities. The GOfax.IP module manages to combine the fax medium, which at first glance seems obsolete, with e-mail effectively and inexpensively in order to guarantee economical communication, especially with many channels.
Fit and sustainable at work – Medat uses JobRad
Cycling is booming! Cycling is mobility, health program, active climate protection and fun rolled into one.
The health of our employees and sustainability are particularly important to us at Medat. That is why we have been offering our team bicycles for commuting and leisure time since the beginning of 2020.
The Freiburg company JobRad GmbH (www.jobrad.de) is a company bike model that we especially like. The principle works in a similar way to leasing a company car: Medat employees can easily and conveniently obtain their desired bike from the company.
Betriebliches Gesundheitsmanagement (BGM) is an important topic in German companies. Just like JobRad, Medat believes that with the help of JobRad employees are happier, more motivated and therefore more productive.
More sustainability and healthy employees are goals that Medat has set itself. At this point we would like to thank all employees for their many years of commitment and great work:
Medat says THANK YOU!
PCR pool tests (lollipop test) in North Rhine-Westphalia
Since May 10, schoolchildren in North Rhine-Westphalia have been tested for the corona virus with so-called “lollipop tests”. These are simple saliva tests that are carried out twice a week in the study groups.
The tests are then carried out by our customer “Labor Dr. Wisplinghoff ”in Cologne.
PCR pool testing is simple and age-appropriate: the students suck on a swab for 30 seconds, similar to a lollipop. It is much more pleasant to perform than throat and nose swabs, and therefore ensures greater acceptance by the participating students.
Finally, the samples from all the children in a study group are brought together and stored as an anonymous collective sample in the “Labor Dr. Wisplinghoff ”analyzed and evaluated. The PCR method provides a very reliable test result.
The tests will initially be carried out until the summer vacation. All primary schools, special schools and schools with primary level in North Rhine-Westphalia have the opportunity to take part in the project.
More information is available at https://schulministerium.nrw/lolli-tests
The path of the sample through the laboratory
Have you ever wanted to know what happens to a blood sample in the laboratory before the results come to you through your doctor?
Our customer, the Staber laboratory, shows in his video the individual steps that a sample takes on its way through the laboratory.
The Medat laboratory information system accompanies the sample at every step. The LIS enables the laboratory to run smoothly, fully electronic data exchange between the individual stations, it analyzes and prepares the information – all fully automated. A laboratory doctor then evaluates the information from the Medat LIS and validates it. Your doctor will then receive the results of the blood sample from the laboratory.
For Medat customers, full automation is not a vision of the future, but a reality today.
You can find more information about the Medat customer Labor Staber on Youtube https://www.youtube.com/channel/UC-zk3__Mhf8GKFFmsQhjWcAand on the websitehttps://www.labor-staber.de/home/
© Dr. Staber & Kollegen GmbH
Medat extends ALM e.V. sustaining membership
Medat has been a sponsoring member of ALM e.V. (Accredited Laboratories in Medicine) for several years.
ALM e.V. represents over 200 accredited partner laboratories in medicine.
As a member of ALM e.V., Medat is networked with numerous medical laboratories and specialists in order to exchange experiences and skills in cooperation. That has proven helpful in the current pandemic.
Medat is effectively helping to shape laboratory diagnostic patient care in Germany and is also always up to date with the latest laboratory medicine standards.
14th BfV / ASW security conference on the topic of “Industrial espionage – a real threat to German companies”
On March 24, 2021, the 14th BfV / ASW security conference took place in digital form with the topic “Industrial espionage – a real threat to German companies”.
As every year, the President of the Federal Office for the Protection of the Constitution, Thomas Haldenwang, opened the event together with the ASW Board Chairman Volker Wagner. Every year over 140 participants from corporate security departments, security authorities and academia come together to discuss the latest developments in the field of corporate security.
This year the managing director of Medat, Stefan Henkelmann, was one of the participants. In a panel discussion with a focus on medium-sized companies, topics such as the security risks of working from home in times of Corona were discussed. In addition to Stefan Henkelmann, guests from various industries were present: Roland Feil (Managing Director of Dallmeier Systems GmbH), Dr. Michael Kilchling (Senior Researcher at the Max Planck Institute) and Christian Kromberg (Administrative Department of the City of Essen).
The security conference was hosted by the Alliance for Security in Business (ASW) and the Federal Office for the Protection of the Constitution (BfV). You can find more information at https://www.asw-bundesverband.de/presse-news/#news_314
Medat at the digital trade fair – DMEA 2021
The corona situation in Germany remains serious. Despite promising advances in vaccines, it is not foreseeable how strongly and how quickly they can actually contribute to a noticeable relaxation, especially with regard to the hosting of trade fairs like the DMEA.
From June 7th to 11th, DMEA 2021 will take place digitally for the first time this year.
The motto of the fair this year is “Connecting Digital Health”: in addition to exclusive webinars and digital tours, the visitor is to be offered an extensive program.
Medat will present itself with a virtual fair stand to give you, as usual, detailed insights into the world of laboratory information systems and to answer your questions.
You can already read the complete program at https://www.dmea.de/de/programm/habenprogramm/. The DMEA ticket shop will shortly go online on the digital platform.
Coronavirus variant: Further innovations in testing
Since January 27, 2021, variant-specific PCR tests have been possible in accordance with the revised Test Ordinance (TestV) of the Federal Ministry of Health (BMG). All persons with a positive PCR test result are eligible – including symptomatic patients who were tested as part of their medical treatment. The aim is to quickly identify the highly infectious SARS-CoV-2 variants.
What should be considered with regard to billing for laboratories and hospitals with the Medat laboratory information system:
If you want to carry out another variant-specific PCR according to TestV in the case of positive PCR tests (regardless of the type of calculation), you only need a unique analyte here. This examination may only be used for billing according to TestV and serves as a trigger in the OEGD dispensing program. The variant-specific examination must be recorded in the same order as the first PCR, otherwise a count is not possible.
The delivery file OEGD (TestV, record type LABTEST) is then created as follows:
If it is an OEGD order in which the normal PCR and the variant-specific PCR are entered
-> type of testing = 3
If the variant-specific PCR is entered in an order with a different accounting type (not TestV)
-> Type of testing = 1 (because the 1st PCR was not billed according to TestV)
Further information can be found on the website of the National Association of Statutory Health Insurance Physicians (KBV) at https://www.kbv.de/html/1150_50494.php
Medat successfully implements new oKFE guidelines
Since October 1st, laboratories with contracted doctors have been documenting the results of evaluated cytological examinations and HPV tests (co-testing) as part of the early detection programs for colon cancer (iFOBT) and cervical cancer.
Medat implements the new oKFE guidelines in your Medat laboratory software. What does that mean for your laboratory?
If you offset the preventive iFOBT with the number 01738, you must now document this in electronic form. In terms of content, this differs only slightly from the previous one. The electronic documentation is required according to § 11 Abs. 4 oKFE-RL Prerequisite for billing services.
If you need the evaluation and data transfer to the KV (health insurance) because you are performing these tests, please contact us promptly by email at abrechnung@medat.de so that we can provide you with the desired programs for documentation.
Corona warning app , Muster 10C and Muster ÖGD
With regard to the new Muster 10C and Muster ÖGD as well as the connection to the Corona warning app, the following adjustments in the system are necessary.
Muster 10C and Muster ÖGD acquisition scan systems
• Extension of the data transfer to include new field identifiers from the KBV defined LDT2.
Order-Entry
• Creation of a new order entry mask with a standard layout.
Graphic acquisition and modification programs
• New laboratory distribution list call with standard layout.
Diagnosis
• The diagnosis remains unchanged.
Report to the health department
• Report findings with standard layout.
Billing Muster 10C
• Quarterly as part of the previous KV billing.
Muster ÖGD
• Monthly via new laboratory distribution list.
Corona warning app
• Export in the following CSV format to suitable interfaces:
reference
referral
Muster ÖGD may not be transferred any further; Muster 10C must be forwarded in the original.
System requirements
Version David 3.7 is a basic requirement; an update to the current release status may be necessary.
Costs
The offer is prepared individually for your system on request
vertrieb@medat.de.
Setup begins after the order is received.